Prof. Dr. Matthias A. Hediger
Membrane Transport Discovery Lab
Head of the Lab
Department of Nephrology and Hypertension & Department of Biomedical Research
Inselspital, University of Bern
Julie-von-Jenner-Haus, Office D812
Freiburgstrasse 15
CH-3010 Bern
Switzerland
Matthias Hediger, Department of Nephrology and Hypertension, University of Bern
matthias.hediger@unibe.ch
Phone: +41 31 632 94 39
Research Goals and Activities
Research arising from the Hediger group has historically focused on the functional characterization of clinically important membrane transporters and ion channels using a variety of methodologies such as electrophysiology, classical transport studies, microscale thermophoresis, structure function analysis, advanced imaging technologies and bioinformatics. Over the past 25 years, this work has revealed the mechanistic foundations for transporters of iron (SLC11A2), vitamin C (SVCT1 and SVCT2), urea (SLC14A2), citrate (SLC13A2), glutamate (SLC1A1), dibasic amino acids (SLC3A1) and peptides (SLC15A1), as well as the epithelial calcium channels TRPV5 and TRPV6. Additional work has been devoted to the mechanisms of store-operated calcium entry by the Stim-Orai pathway. Most recent efforts are being devoted towards deciphering biological and pharmaceutical aspects of the SARS-CoV-2 virus pandemic.
Current projects
- The SLC-ome of membrane transport
- New insights into the COVID-19 pandemic: Genetic polymorphisms, role of SLC6 amino acid transporters, renal aspects and therapeutic perspectives
- Calcium channel TRPV6 in health and disease
- Structure, function and pharmacology of Orai/STIM calcium channels
- Mitochondrial carriers and regulation by calcium signaling
- Structure, function and therapeutic implications of the SLC11A2/DMT1 iron transporter
- SLC39/ZIP metal ion transporters and roles in pathologies
- Role of amino acid transporters in colorectal cancer progression
- Investigation of the biology of the endosomal peptide/histidine transporter SLC15A4
- Glial glutamate transporters (SLC1A2/GLT1)
Other activities
Current Projects
1. The SLC-ome of membrane transport
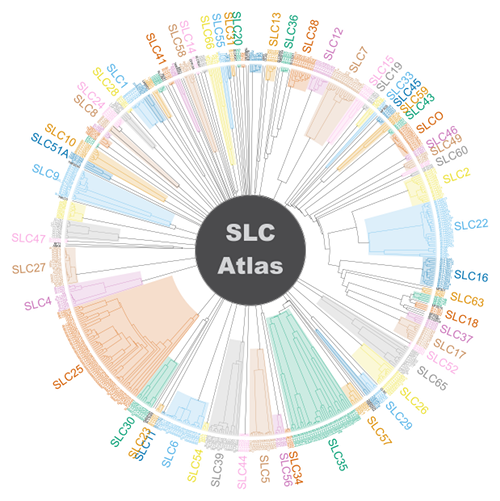
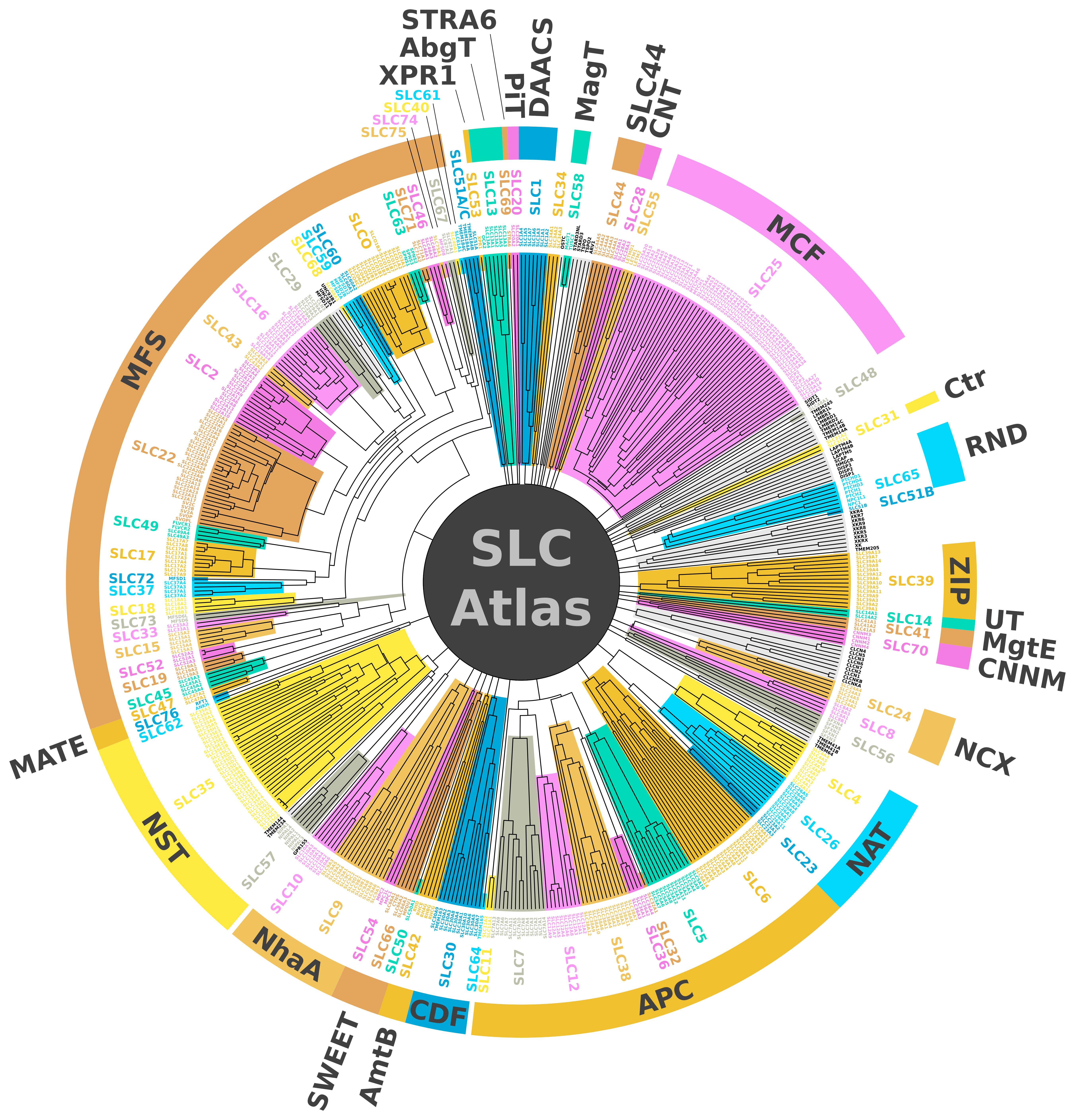
The Membrane Transport Discovery Lab (Group Leader: Prof. em. Matthias A. Hediger; https://www.bioparadigms.org/) investigates membrane transport proteins that are essential for human physiology and disease. These transporters act as molecular gatekeepers at cellular and organellar membranes, and their dysfunction contributes to a wide range of pathological conditions. A central focus of our work is the systematic identification, classification, and functional annotation of solute carrier (SLC) transporters in mammalian genomes. Approximately 5.2% of the human protein-coding genome encodes membrane transport proteins, and nearly half of these—currently about 464 genes—belong to the SLC superfamily. Collectively, this entire set of secondary and facilitative transporters, excluding ATP-driven pumps, ABC transporters, ion channels, and aquaporins, is referred to as the SLC-ome. The human SLC-ome currently comprises nearly 500 genes organized into 76 families defined by shared sequence features, conserved structural folds, and related transport mechanisms. By integrating publicly available databases with curated literature analysis, we have contributed to the discovery and organization of previously unrecognized SLC candidates, visualized in the SLC Atlas wheel shown on the right, which provides a comprehensive overview of SLC family relationships. SLC transporters are expressed across virtually all tissues and organ systems and are essential for processes such as nutrient uptake, ion and metabolite homeostasis, development and growth, intracellular signaling, immune defense, and nervous system function. In addition, approximately 50 proteins with clear SLC-like features have been identified that are not yet fully characterized or formally incorporated into the SLC nomenclature, representing a substantial and promising frontier for future discovery in membrane transport biology. Refs.: César-Razquin A. et al., Cell (2015); Gyimesi et al., Physiological Reviews (2024).
2. New insights into the COVID-19 pandemic: Genetic polymorphisms, role of SLC6 amino acid transporters, renal aspects and therapeutic perspectives
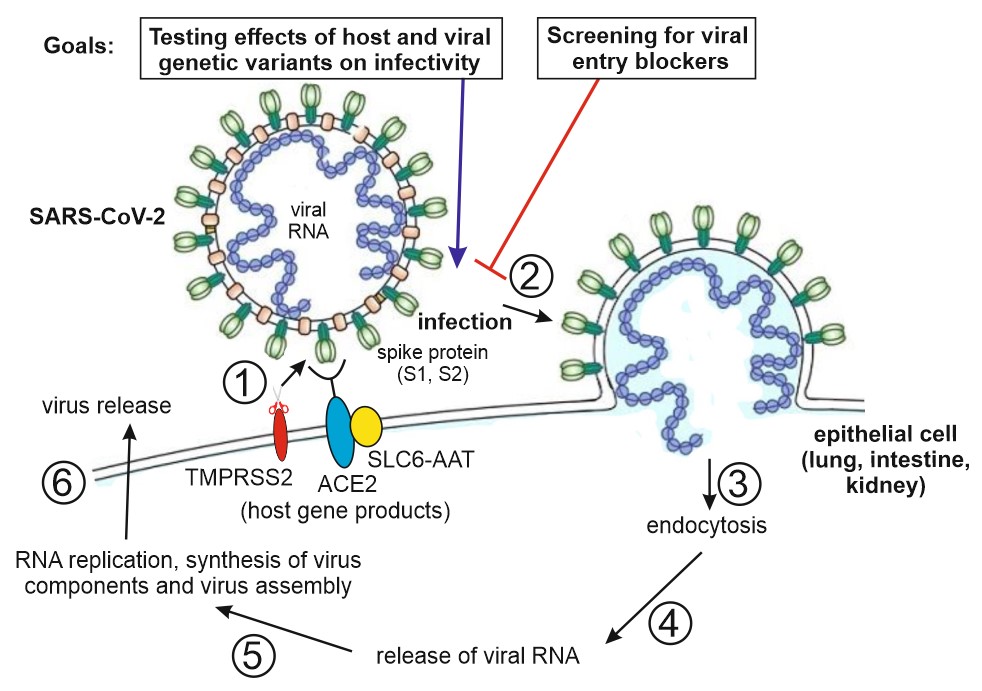
This project aims to decipher biological and pharmaceutical aspects of the SARS-CoV-2 virus pandemic. Using a combination of biochemical assays such as micro-scale thermophoresis (MST) to determine SARS-CoV-2 receptor binding domain (RBD) binding affinity to the ACE2 virus receptor and the SARS-CoV-2 pseudovirus entry assay to reveal viral load, we will clarify the roles of specific allelic variants of viral host genes in conferring COVID-19 severity. In addition, we will screen for blockers of viral susceptibility as hit/lead compounds for the development of novel treatment strategies.
The figure on the right shows the steps involved in SARS-CoV-2 infection in epithelial cells of lung, intestine and kidney, and project strategy outline. Using a combination of binding and pseudovirus entry assays, we will determine the effects of genetic host and viral variants on virus infectivity. Furthermore, we will screen for blockers of viral infection that may serve as future hit/lead compounds for the development of novel antiviral therapies, e.g. by administering identified agents using an oral or nasal spray, as an alternative to COVID-19 vaccination. Abbreviations: ACE2, angiotensin-converting enzyme 2 (serves as virus receptor); TMPRSS2, transmembrane protease (activates SARS-CoV-2); SLC6-AAT, SLC6 family amino acid transporter (transports peptide cleavage products of ACE2 into epithelial cells).
3. Calcium channel TRPV6 in health and disease
As part of the initial activities of the NCCR TransCure network, we have been working with the group of Jean-Louis Reymond to generate specific TRPV6 inhibitors. Our effort has led to the generation of a series of novel inhibitors with great specificity and IC50 values in the nanomolar range (Angew Chem Int Ed Engl. 2015, 54:14748-52; RSC Med Chem 2020, 11, 1032-1040). In our recent collaboration with Alexander Sobolevsky (University of Columbia) and Christoph Romanin (University of Linz), we combined structural data with mutagenesis and functional/computational analyses to clarify the binding site of these compounds within the open pore of TRPV6. Our study shows that binding converts the channel into a nonconducting state, thereby mimicking the action of calmodulin, which causes inactivation of TRPV6 channels under physiological conditions. This mechanism of inhibition explains the high selectivity and potency of these channel inhibitors and opens up unexplored avenues for the design of future-generation biomimetic drugs (Sci Adv. 2020 Nov 27; 6). Furthermore, in collaboration with Jean-Louis Reymond and Christoph Romanin, we developed a novel photoswitchable inhibitor of TRPV6 that is expected to serve as a versatile tool compound to deepen our understanding of TRPV6 (ACS Med Chem Lett. 2019; 10:1341-1345 ). In addition, we published a paper on capsaicin derivatives and their effects on TRPV1 and TRPV6 channel function (Bioorg Med Chem. 2019; 27:2893-2904). In addition, a review entitled “TRPV5 and TRPV6 Calcium-Selective Channels” was published as a book chapter in “Calcium Entry Channels in Non-Excitable Cells” (Kozak JA, Putney JW Jr., editors, CRC Press/Taylor & Francis; 2018; Chapter 13).
4. Structure, function and pharmacology of Orai/STIM calcium channels
In year 2015, our laboratory got awarded the Sinergia Swiss National Science Foundation (SNSF) interdisciplinary research grant, entitled “Store-operated calcium channels in health and disease” (October 1, 2015–November 30, 2018), together with Nicolas Demaurex (University of Geneva) and Martin Lochner (University of Bern). The first study on this topic we published in collaboration with Nicolas Demaurex, showing that ORAI1 channel gating and selectivity is differentially altered by natural mutations in the first or third transmembrane domain (J Physiol. 2019 597:561-582). Thereafter, as a collaboration with Christoph Romanin, we reported the unveiling of a novel STIM1-Orai1 gating interface that is essential for CRAC channel activation (Cell Calcium. 2019; 79: 57-67). In collaboration with Martin Lochner, we characterized novel 2-aminoethyl diphenylborinate (2-APB) derivatives with respect to inhibition of store-operated calcium entry (SOCE) (Int J Mol Sci. 2020 Aug 5; 21(16):5604). In another recent study, we described a novel role for Ca2+-calmodulin in SCDI of Orai1 (Cell Physiol Biochem 2020; 54:252-270). In addition, we completed our collaboration with Ivan Bogeski (University of Göttingen) and Rainer Schindl (University of Graz), showing that STIM2 cytosolic cysteine residues are targeted by reactive oxygen species to modulate SOCE (Cell Rep. 2020; 33(3):108292). Related to this work, we published a review entitled “Redox modulation of STIM-ORAI signaling” (Cell Calcium. 2016; 60:142-52).
5. Mitochondrial carriers and regulation by calcium signaling
In 2018, we received a new SNSF Sinergia grant to study the role of mitochondrial carriers in metabolic tuning and reprogramming by calcium flow across membrane contact sites, a collaborative effort together with Edmund Kunji (University of Cambridge) and Martin Lochner. A review entitled “Sequence Features of Mitochondrial Transporter Protein Families” has recently been published (Biomolecules. 2020 28; 10(12):E1611).
6. Structure, function and therapeutic implications of the SLC11A2/DMT1 iron transporter
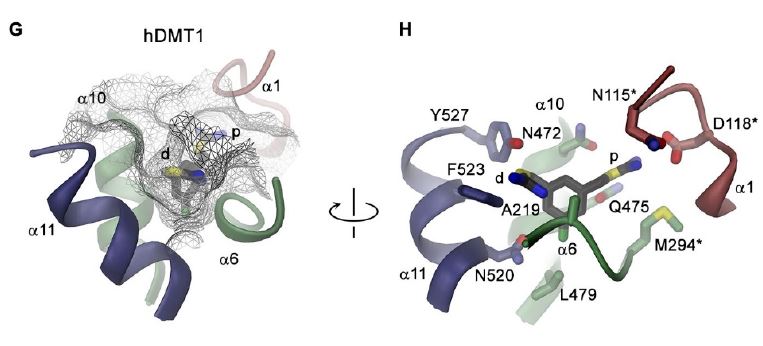 After the molecular discovery of the divalent metal ion
transporter by our group (Nature. 1997, 388:482-8), Raimund
Dutzler, as part of the NCCR TransCure iron project, reported the
3D structure of DMT1/SLC11A2 from Staphylococcus capitis
(ScaDMT), unveiling it as a LeuT-fold transporter. As a
follow-up, we employed molecular dynamics simulations and
site-directed mutagenesis and discovered a novel H+ transfer
mechanism in DMT1. Our molecular dynamics simulations provided
first insight into how H+-translocation through E193 is
allosterically linked to intracellular gating, revealing a novel
H+-coupling mechanism that is distinct from that of other
H+-transporters (Sci Rep. 2017 ;7:6194). The mechanistic base of
the inhibition of DMT1 has recently been elucidated for
bis-isothiourea substituted compounds in collaboration with the
groups of Raimund Dutzler (University of Zürich) and Jean-Louis
Reymond (University of Bern) (eLife 2019; 8: e51913).
After the molecular discovery of the divalent metal ion
transporter by our group (Nature. 1997, 388:482-8), Raimund
Dutzler, as part of the NCCR TransCure iron project, reported the
3D structure of DMT1/SLC11A2 from Staphylococcus capitis
(ScaDMT), unveiling it as a LeuT-fold transporter. As a
follow-up, we employed molecular dynamics simulations and
site-directed mutagenesis and discovered a novel H+ transfer
mechanism in DMT1. Our molecular dynamics simulations provided
first insight into how H+-translocation through E193 is
allosterically linked to intracellular gating, revealing a novel
H+-coupling mechanism that is distinct from that of other
H+-transporters (Sci Rep. 2017 ;7:6194). The mechanistic base of
the inhibition of DMT1 has recently been elucidated for
bis-isothiourea substituted compounds in collaboration with the
groups of Raimund Dutzler (University of Zürich) and Jean-Louis
Reymond (University of Bern) (eLife 2019; 8: e51913).
7. SLC39/ZIP metal ion transporters and in pathologies
A combination of in silico and in vitro techniques involving structural modeling, mutagenesis and functional characterization was employed to unravel the structural elements of pH sensitivity and substrate binding in the human zinc transporter SLC39A2 (ZIP2). This work provides the first structural evidence for the previously observed pH and voltage modulation of ZIP2-mediated metal transport (J Biol Chem. 2019; 294:8046-8063). An inhibitor against the SLC39A8/ZIP8 zinc transporter has recently been generated and is being tested using an in vitro osteoarthritis cellular system, looking for beneficial disease treatment effects.
8. Role of amino acid transporters in colorectal cancer progression
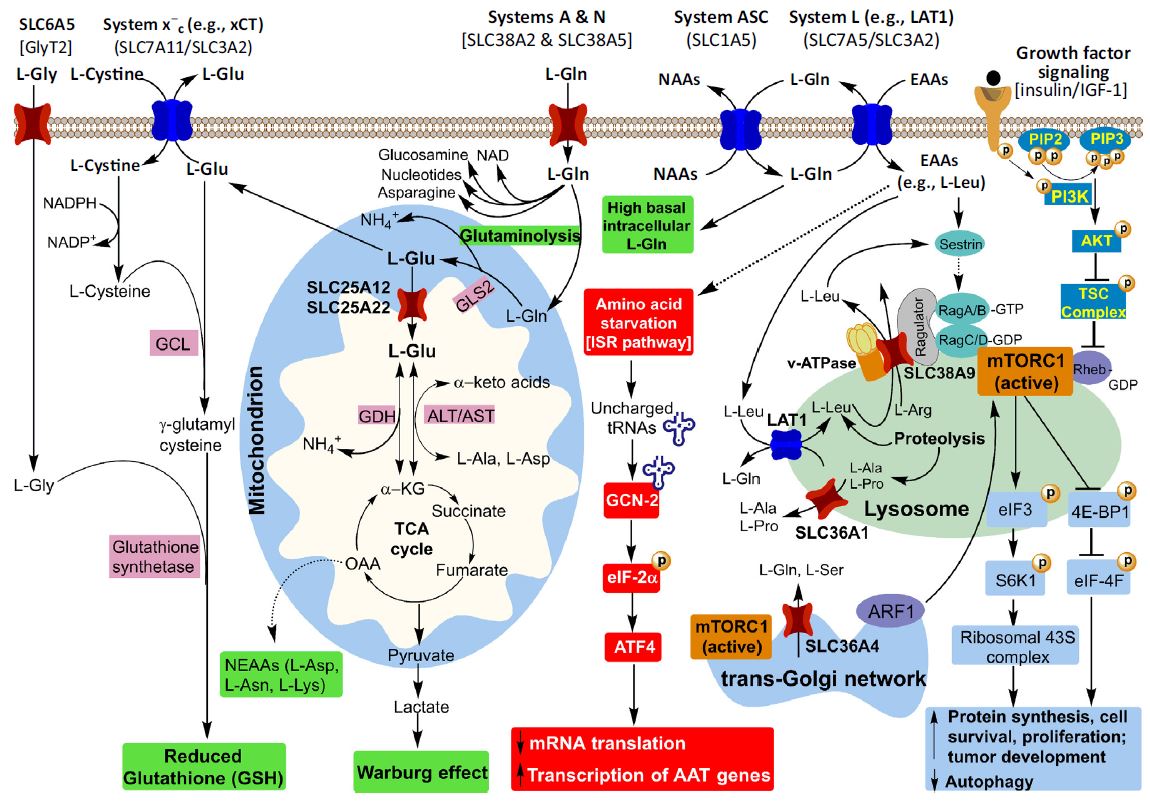 The figure on the right shows the role of amino acid transporters
in mTOR activation, energy metabolism, nutritional stress and
tumor progression (Trends Biochem Sci. 2018, 43(10):752-789). Our
recent data reveal that oncogenic mutations boost amino acid
delivery into colorectal cancer (CRC) cells via hippo-mediated
upregulation of specific amino acid transporters (manuscript in
preparation). Briefly, we investigated the impact of oncogenic
and tumor suppressor mutations on the regulation of expression of
amino acid transporters in CRC. We found that KRAS and BRAF
oncogenic mutations present in different CRC subtypes upregulate
the expression and functions of specific amino acid transporters.
Our findings suggest that inhibiting amino acid transporters
could represent a novel approach for personalized cancer
treatment in KRAS/BRAF-mutant tumors.
The figure on the right shows the role of amino acid transporters
in mTOR activation, energy metabolism, nutritional stress and
tumor progression (Trends Biochem Sci. 2018, 43(10):752-789). Our
recent data reveal that oncogenic mutations boost amino acid
delivery into colorectal cancer (CRC) cells via hippo-mediated
upregulation of specific amino acid transporters (manuscript in
preparation). Briefly, we investigated the impact of oncogenic
and tumor suppressor mutations on the regulation of expression of
amino acid transporters in CRC. We found that KRAS and BRAF
oncogenic mutations present in different CRC subtypes upregulate
the expression and functions of specific amino acid transporters.
Our findings suggest that inhibiting amino acid transporters
could represent a novel approach for personalized cancer
treatment in KRAS/BRAF-mutant tumors.
9. Investigation of the biology of the endosomal peptide/histidine transporter SLC15A4
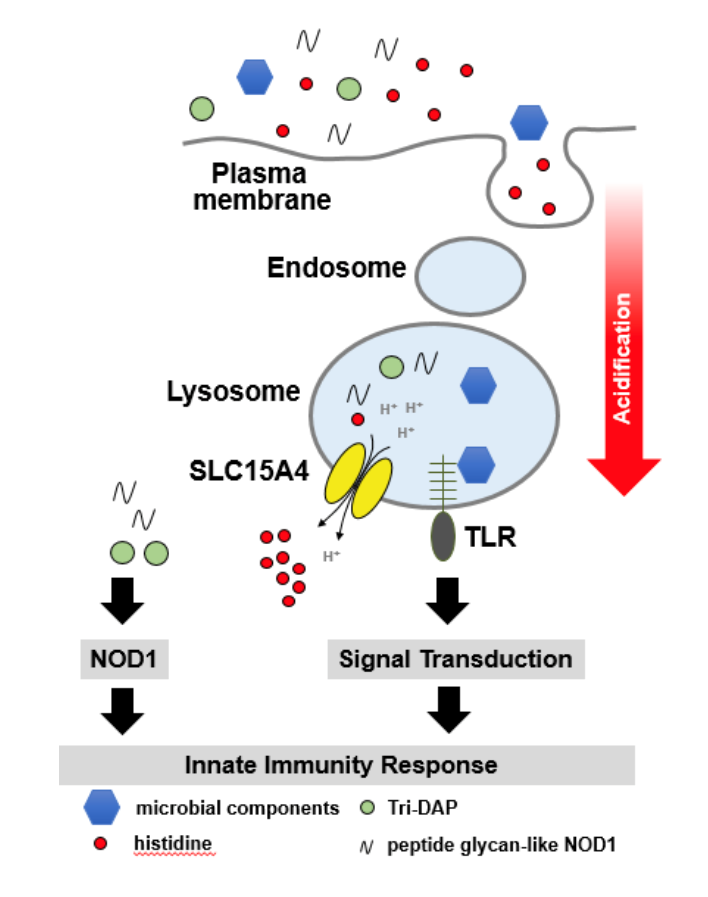 The orphan endosomal peptide/histidine transporter SLC15A4 is
currently being evaluated with respect to application to the
treatment of autoimmune diseases such systemic lupus
erythematosus (SLE) and inflammatory bowel disease (IBD). The
SLC15 family includes four different members (SLC15A1-A4).
SLC15A1 (intestinal PepT1) and SLC15A2 (renal PepT2) have
previously been extensively studied by our laboratory
(Nature. 1994, 368(6471):563-6).
SLC15A3 and SLC15A4 have low sequence similarities to
SLC15A1 and SLC15A2 and their substrate selectivity is thought to
be restricted to histidine, certain oligopeptides and muramyl
peptides that represent fragments of peptidoglycan from
pathogens, e.g. the bacterial cell wall. SLC15A4 is a
peptide/histidine transporter essential component of the
inflammatory response system triggered by Toll-like receptors
(TLR) pathway. Ligand binding and signaling of TLRs are modulated
by the content of histidine and the pH inside the lysosomes (see
Figure). The histidine level in lysosomes is thought to be
maintained within a defined concentration range by SLC15A4 to
warrant maximal functional efficiency of the lysosomal
components, including TLR maturation and function. The absence of
SLC15A4 leads to failure in the homeostasis of the lysosomal
environment, which could explain the disruption of the TLR
signaling pathway in SLC15A4-deficient cells. Additionally,
SLC15A4 seems to be involved in the transport of the
peptidoglycan-like NOD1 ligand, tri-DAP, and the NOD2 cognate
ligand muramyl dipeptide (MDP) from lysosomes to the cytosol.
Our goal is to generate SLC15A4 inhibitors as a therapeutic
treatment strategy for SLE and IBD. Using microscale
thermophoresis (MST), we have screened a diverse library of 1600
compounds and identified several hits that are currently being
investigated.
The orphan endosomal peptide/histidine transporter SLC15A4 is
currently being evaluated with respect to application to the
treatment of autoimmune diseases such systemic lupus
erythematosus (SLE) and inflammatory bowel disease (IBD). The
SLC15 family includes four different members (SLC15A1-A4).
SLC15A1 (intestinal PepT1) and SLC15A2 (renal PepT2) have
previously been extensively studied by our laboratory
(Nature. 1994, 368(6471):563-6).
SLC15A3 and SLC15A4 have low sequence similarities to
SLC15A1 and SLC15A2 and their substrate selectivity is thought to
be restricted to histidine, certain oligopeptides and muramyl
peptides that represent fragments of peptidoglycan from
pathogens, e.g. the bacterial cell wall. SLC15A4 is a
peptide/histidine transporter essential component of the
inflammatory response system triggered by Toll-like receptors
(TLR) pathway. Ligand binding and signaling of TLRs are modulated
by the content of histidine and the pH inside the lysosomes (see
Figure). The histidine level in lysosomes is thought to be
maintained within a defined concentration range by SLC15A4 to
warrant maximal functional efficiency of the lysosomal
components, including TLR maturation and function. The absence of
SLC15A4 leads to failure in the homeostasis of the lysosomal
environment, which could explain the disruption of the TLR
signaling pathway in SLC15A4-deficient cells. Additionally,
SLC15A4 seems to be involved in the transport of the
peptidoglycan-like NOD1 ligand, tri-DAP, and the NOD2 cognate
ligand muramyl dipeptide (MDP) from lysosomes to the cytosol.
Our goal is to generate SLC15A4 inhibitors as a therapeutic
treatment strategy for SLE and IBD. Using microscale
thermophoresis (MST), we have screened a diverse library of 1600
compounds and identified several hits that are currently being
investigated.
10. Glial glutamate transporters (SLC1A2/GLT1)
Our recent collaboration with Rodan, Lance, Children’s Hospital, Harvard Medical School revealed novel glutamate transporter mutations that cause epilepsy via a dominant negative mechanism. We found that these mutations are localized in the trimerization domain of the glutamate transporter trimers (Ann Neurol. 2019, 85:921-926).
1. Establishment of NCCR TransCure
The National Centre of Competence in Research (NCCR) “TransCure” has been initiated and directed by Matthias Hediger starting in year 2010, with the main goal to accelerate the transformation of knowledge from basic research within the transporter/channel field into use. The leading house of this network is the University of Bern (www.nccr-transcure.ch/about-us, and it is currently ran by Hugues Abriel. Our network is composed of a dozen multidisciplinary scientific laboratories affiliated at Universities in Basel, Bern, Lausanne and Zürich. This team of Swiss academic experts are focused on cellular membrane transporter/channel research and emerging applications for the treatment of human diseases. Using TransCure's approach “from transport physiology to the identification of therapeutic targets” researchers are working collaboratively, combining a unique interdisciplinary skill-set that encompasses three major disciplines, referred to as “TransCure Trias”: Physiology/Medicine, Structural Biology and Medicinal Chemistry. The NCCR TransCure projects substantially advanced our understanding of the physiological roles of SLC transporters and helped reveal the largely untapped therapeutic potential of the human SLC-ome, paving the way for the development of novel therapeutic strategies to improve human health. (Ref: Rossetti V. & Abriel H., Chimia, 2022)
2. Conference organization
Matthias Hediger has established two different international conference series to promote the transporter/ion channel field in the biomedical and pharmaceutical areas:
- International Gordon Research Conference Series on transporters and channels. The next Conference of this series will be held from June 12-17, 2022 in Castelldefels, Spain and chaired by Aurelio Galli.
- International BioMedical Transporter Conference Series (held biennially) on the pharmaceutical aspects of transporters and channels. The focus is on transporter and ion channel-based drug discovery strategies, pharmacokinetics, drug delivery and drug elimination. As a result of the COVID-19 outbreak, the format and time schedule for the future BioMedical Transporters Conferences is currently being reassessed.

Dr. Gergely Gyimesi
Postdoc
gergely.gyimesi@unibe.ch

Dr. Palanivel Kandasamy
Postdoc
palanivel.kandasamy@unibe.ch